Manual Muscle TesterMobie ZⅡ
Manual Muscle TesterMobie ZⅡ

A compact, lightweight muscle strength tester.
A high-precision, push-type manual muscle strength tester.
It can accurately measure muscle strength using the thrust sensor, which is often difficult to convey to the person being tested in MMT3, 4, and 5 (also displayable in Kgf, N, or lbs).
It uses a replaceable nickel-metal hydride rechargeable battery.
The Mobie main unit can be charged via USB with an AC power source and used while charging, even when the battery is low. It uses AA-size nickel-metal hydride rechargeable batteries, so you can quickly replace the battery with a fully charged one.
Key features
Wireless digital data transmission is available.

By transmitting muscle strength data digitally to a PC, you can evaluate not only maximum muscle strength (peak/value) but also time factors, muscle fatigue (time variation), and muscle effort adjustment.
The maximum measurable force is 150 kgf.
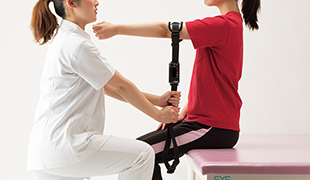
By exchanging the tension sensor, traction method measurements can also be conducted. Since it is fixed using a strap, areas that are difficult to measure manually can be easily assessed.
Pinch force can be measured in 0.1 kgf increments using a pinch force sensor.

By using the pinch force sensor, pinch strength can be measured in 0.1 kgf units.
The 5-position test and the standard 2-position test are made easier.

By replacing the sensor with the overseas standard Jamar-type handle, the 5-position test and standard 2-position tests can be easily performed.
Mobie ZⅡ software
This software is dedicated software for the "Handheld Muscle Strength Tester / MobieZⅡ"
(hereinafter referred to as MobieZⅡ).
It displays and records muscle strength (values) by receiving electronic data sent from MobieZⅡ via WiFi or USB (sampling rate 100Hz).
※When downloading the software, please be sure to review the following terms of use.
※Downloading the software means you agree to the terms of use.
※The downloaded file needs a password for extraction. The password is provided in the user manual that comes with the product at the time of purchase.
【MobieZⅡ Software Terms of Use】
The following terms (hereinafter referred to as "Terms") constitute the agreement between the customer (hereinafter referred to as "User") and Sakai Medical Co., Ltd. regarding the use of this software.
By installing or using this software, you agree to these terms. If you do not agree to these terms, do not install or use this software.
Article 1 (General Rules)
Sakai Medical Co., Ltd. grants the User the right to use this software, subject to the agreement to these terms.
Article 2 (Usage Rights)
1.Under this agreement, Sakai Medical Co., Ltd. grants the User a non-exclusive, non-transferable right to use this software.
2.The User may not copy, rewrite, modify, add, disassemble, or perform any other actions on the whole or part of this software without the prior written consent of Sakai Medical Co., Ltd. Furthermore, the User may not transfer or resell the rights granted under this agreement to any third party.
Article 3 (Guarantee)
1. Sakai Medical Co., Ltd. does not guarantee that the service will be error-free, nor does it guarantee that the information provided through this software is complete, accurate, usable, or applicable for any specific purpose of the User.
2.In the event of a major defect in the software, Sakai Medical Co., Ltd.'s responsibility for repair is limited to correcting or eliminating the defect within commercially reasonable limits.
Article 4 (Disclaimer by Sakai Medical Co., Ltd.)
The User assumes all risks associated with the use of this software. Sakai Medical Co., Ltd. is not liable for any damages caused by information created based on the usage rights provided under this agreement.
Article 5 (Liability to Third Parties)
If the use of this software infringes upon copyrights, patents, or other intellectual property rights and leads to a dispute with a third party, the User shall be responsible for resolving the dispute and shall not cause any inconvenience to Sakai Medical Co., Ltd.
Article 6 (Other)
1.Even if certain provisions of this agreement are found to be invalid by specific laws, the remaining provisions of the agreement shall continue to be valid.
2.The User may not export this software in violation of the export control laws of the relevant country.
3.This agreement shall be governed and interpreted in accordance with Japanese law.
When using this software, the User is responsible for reading, understanding, and agreeing to these terms. In addition, the User agrees that these terms are complete and exclusive for the exchange of software between Sakai Medical Co., Ltd. and the User, whether in title or written form, and that communication during any stage with the User or any third party shall not invalidate this agreement.
September 9, 2021
Sakai Medical Co., Ltd.
Click here to download the software
【Mobie Software】
Recommended Software Operating Environment
【OS】:Windows10
【CPU】:intel® Core i5以上
【内存】:8GB以上
【HDD】:500GB以上
※If you have any questions, please contact us.
Specifications
Mobile ZⅡMT-202

| Main Body Dimensions | 90(L)×48(W)×32(H)㎜ |
|---|---|
| Main Body Weight | Approx.240g(display unit + push force sensor) |
| Measurement Range | Up to 50kgf |
| Minimum Unit | 0.1kgf |
| Accuracy | ±2% RO or below |
| Display Units (switchable) | kgf, N (Newton), lbf (pound-force) |
| Digital Data Transmission | 〇 ※Wireless and USB transmission: Can switch between both ※Dedicated Software: Included (Requires PC with Windows 10 OS) |
| Analog Output Function | 〇 Note: Requires separately purchased dedicated cable |
| Continuous Usage Time | 40 minutes (Charging time: 1.5 hours) |
| Components | Display unit, push force sensor, cushioning pad, wrist strap Nickel-hydride rechargeable battery (size AA) x 1, USB charging cable Note: Only use nickel-hydride rechargeable batteries. |
【Optional Accessory】Tension SensorMT-250

| Main Body Dimensions | 114(L)×48(W)21(H)㎜ |
|---|---|
| Strap Dimensions | 1000(L)×24(W)㎜(excluding buckle) |
| Main Body Weight | Approx. 145g |
| Measurement Range | Up to 150kgf |
| Minimum Unit | 0.1kgf |
【Optional Accessory】Pinch Force SensorMT-240
.jpg)
| Main Dimensions | 115(L)×45(W)×17(H)㎜ |
|---|---|
| Main Weight | Approx. 160g |
| Pinch Plate Width | Approx. 10mm |
| Measurement Range | Up to 30kgf |
| Minimum Unit | 0.1kgf |
【Optional Accessory】Grip Force SensorMT-230

| Main Body Dimensions | 175(L)×140(W)×35(H)㎜ |
|---|---|
| Main Body Weight | Approximately 490g |
| Measurement Range | Up to 90kgf |
| Minimum Unit | 0.1kgf |
【Optional Accessory】Storage BoxMST-10

| Main Body Dimensions | 350×260×90 ㎜ |
|---|---|
| Usable Dimensions | 340×180×70 ㎜ |
| Weight | Approximately 900g |
| Weight Capacity | 5 ㎏ |
| Material | High-Density Polyethylene |
【Optional Accessory】Analog Output CableMT-221
| Cable Length | Approximately 1000 mm |
|---|---|
| Connector | USB( micro-B) to BNC(Male) |
※When outputting data to a computer, A/D conversion and other processes are required.
Related Information
-
The goal is to establish a muscle strength standard based on 10,000 people.
Most importantly, every therapist has an HHD in their pocket. Objective measurements using the HHD are becoming a regular part of daily practice.
Continue reading (Japanese)